An oncology physician practice who did business with Cardinal Health paid the United States and the State of Michigan agreed to pay $1 million to resolve allegations that the practice accepted kickbacks from Cardinal in connection with the purchase of specialty pharmaceutical products from Cardinal. The physician practice was one of several such practices sued along with Cardinal Health. In January 2022, Cardinal Health agreed to pay the United States and the states $13.125 million plus interest to resolve allegations that it induced physician practices such as this one to purchase specialty pharmaceutical products from it by paying customers remuneration in advance of the practice making any drug purchases and not in connection with specific purchases. The practices in turn submitted claims for payment to Medicare and Medicaid programs that were tainted by these kickbacks. Litigation is ongoing as to several other named physician practice defendants.
Under the Anti-Kickback Statute, 42 U.S.C. § 1320a-7b(b), both sides of a kickback arrangement bear liability. It is important to hold both sides of the alleged kickback scheme (here, the payor/Cardinal and the payees/physician practices) accountable. Using the False Claims Act to do so protects the public fisc from paying claims tainted by kickbacks and protects patients. As the U.S. Attorney for the District of Massachusetts said at the time of the Cardinal settlement:
Kickback schemes, such as this one, have the potential to pervert clinical decision-making and are detrimental to our federal health care system and taxpayers.
Special Agent in Charge of the FBI Boston office added at the time: “Kickbacks cost health benefit programs millions of dollars in potentially fraudulent claims.”
Whistleblower Law Collaborative LLC is based in Boston. It devotes its nationwide practice to representing whistleblowers bringing actions under the federal and state False Claims Acts and other whistleblower programs. Under the False Claims Act, a private citizen who knows of fraud against the government can file a sealed complaint on behalf of the government. If the case is successful, the relator is entitled to a share of the government’s recovery. Among the firm’s many successes is the government’s $885 million settlement with AmerisourceBergen, another pharmaceutical drug wholesaler, for illegal repackaging of injectable drugs into pre-filled syringes.
In October, 2022, the United States settled a False Claims Act case brought one of our clients against DermaTran Health Solutions, LLC, a compounding pharmacy based in Rome, Georgia, its parent State Mutual Insurance Company, President Delos Yancey, and other defendants. Under the Agreement, Defendants agreed to pay over $6.87 million to resolve allegations that they violated the False Claims Act by waiving copays, charging the government higher prices than permitted, and trading federal healthcare business with other pharmacies.
As alleged in our Complaint,Yancey caused State Mutual Insurance subsidiaries to bankroll DermaTran Health Solutions, LLC, to sell “compounded” pain creams. Compounded drugs are drugs created by a pharmacy pursuant to a doctor’s order. These are often described as “custom” made formulations, but the vast majority of DermaTran’s compounds consisted of a very few formulations. Another State Mutual Insurance Company subsidiary named Pharmacy Insurance Administrators, LLC (“PIA”), was created to handle the billing for DermaTran.
Compound pain creams were very lucrative. Government-backed health insurance programs such as TRICARE (for the military) and the Federal Employees Health Benefits Program (for federal workers) would often reimburse thousands of dollars for these prescriptions. But the government programs imposed certain restrictions to limit spending. Most notably, these programs require that pharmacies charge them no more than the “usual and customary price”—or the price it would charge to cash-paying, uninsured patients.
In the interests of profit, the defendants ignored these restrictions. For example, they would represent to TRICARE that the hundreds or thousands of dollars they charged veterans was the “usual and customary” price for that drug. However, defendants regularly, and often the same day, sold the exact same prescription for as little as $30 when a patient’s insurance would not pay. Similarly, defendants utilized various schemes to avoid charging copays to patients to make them indifferent to the high prices they charged insurers. In some instances, DermaTran sales personnel offered and paid kickbacks to doctors to induce them to write prescriptions to the pharmacy.
Eventually, insurers began to terminate DermaTran from their networks. To keep the scheme alive, DermaTran, began transferring prescriptions to other pharmacies which kicked back part of the proceeds to DermaTran. Three other pharmacies, also named as defendants, participated in this prescriptions-trading scheme and settled claims: Legends Pharmacy (in Texas), Lake Side Pharmacy (in Alabama), and TriadRx (in Alabama).
Under the terms of the False Claims Act settlement, PIA contributed $6.5 million plus interest to the settlement. It also paid damages to our client for retaliating against her, which is illegal under the FCA.
DermaTran was sold to a third-party, the proceeds of that sale were also turned over to the government as part of the settlement. Legends Pharmacy will pay $59,293. TRIAD Rx, Inc. will pay $166,547. Lake Side Pharmacy is no longer in business, but former owners of Lake Side Pharmacy will pay $110,724.
The affected Government Agencies praised the settlement and thanked our client for coming forward:
Waiving copays and charging the government higher prices leads to overutilization and costs federal programs millions of dollars in unnecessary spending, Our office will continue to enforce the False Claims Act to recover government payments that result from such misconduct.
Northern District of Georgia U.S. Attorney Ryan Buchanan.
Health care fraud abuse like this case erodes the trust patients have in the health care system, the FBI will not stand by when there are allegations of companies operating corporate wide schemes to illegally line their pockets.
Keri Farley, Special Agent in Charge of FBI Atlanta.
Fraud through compounding pharmacies bilked billions out of TRICARE and undermined the integrity of our healthcare system designed to care for our service members and their families, I appreciate the partnership among involved law enforcement agencies and the U.S. Attorney’s Office to bring this matter to justice.
Cynthia Bruce, Special Agent in Charge of the Department of Defense, Office of Inspector General, Defense Criminal Investigative Service (DCIS).
The OPM OIG has no tolerance for businesses that knowingly take advantage of FEHBP, violating the rules to make a profit, I am extremely proud of the hard work of our investigators, analysts, and other law enforcement partners because overcharging the government is not a victimless crime – it contributes to higher premium prices and harms the financial integrity of the FEHBP.
Amy K. Parker, Special Agent in Charge, OPM OIG.
The U.S. Postal Service, Office of Inspector General, will continue to tirelessly investigate those who commit frauds against federal benefit programs and the U.S. Postal Service. This settlement is a clear message that the USPS OIG is dedicated to rooting out corruption and bringing to justice those responsible for these crimes, The USPS OIG would like to thank our law enforcement partners and the Department of Justice for their efforts in this investigation.
Special Agent in Charge Matthew Modafferi of the U.S. Postal Service, Office of Inspector General Northeast Area Field Office.
Health care providers that try to boost their profits by submitting fraudulent claims to Federal health care programs threaten the integrity of those programs and drive up prices for everyone, we work tirelessly alongside our law enforcement partners to protect the integrity of Federal health care programs and to ensure the appropriate use of taxpayer dollars.
Tamala E. Miles, Special Agent in Charge with the U.S. Department of Health and Human Services Office of Inspector General.
Our client was hired by State Mutual Insurance Company to provide accounting services for Dermatran. While there, she repeatedly expressed concerns about the practices she witnessed. However, after being repeatedly ignored by her employers she approached our firm to ask for assistance in providing government prosecutors with information about the alleged fraud. In 2017, we filed a complaint under the federal False Claims Act.
Under the False Claims Act, a private citizen (known as a “relator”) who suspects or knows of fraud against the government can act as a whistleblower and file a sealed complaint on behalf of the government. If the case is successful, the relator is entitled to a share – between 15% and 30% – of the government’s recovery.
In this case, our client will receive $1,434,775 or nearly 21% of the funds received by the government in addition to a payment from defendants as compensation for their retaliation against her.
The process of this suit was long, stressful and sometimes scary – but it was necessary. When you see people in business who take advantage of the system, without regard to the harm it causes to veterans, hard-working citizens, and taxpayers, you can’t stand by silently. I am grateful to my amazing legal team, for standing by my side throughout.
As this case illustrates, whistleblowers are a critical part of fraud enforcement. Last year, according to DOJqui tam cases resulted in over $1.6 billion in False Claims Act recoveries. The False Claims Act is one of the government’s most powerful tools to combat health care fraud.
Whistleblower Law Collaborative LLC attorney David W. S. Lieberman praised the outstanding work of Assistant U.S. Attorneys Anthony DeCinque, Neeli Ben-David, and Armen Adzhemyan. “This was an extremely complex fraud, but the attorneys in the Northern District of Georgia worked hand in glove with relator to understand the scheme and bring the case to a successful resolution.”
WLC attorneys Bruce Judge and Suzanne Durrell added their appreciation for WLC’s courageous client who sounded the alarm on this important issue. “Our client could easily have kept silent about the fraud she witnessed, but she did the right thing and came forward to tell the government about a serious fraudulent scheme that was draining millions from veterans and other vulnerable patients. We view this settlement as a clear vindication of her difficult choice to come forward.”
The Whistleblower Law Collaborative is also grateful for the assistance provided by our co-counsel, Julie Bracker and Jason Marcus of Bracker and Marcus and Joshua Russ and Allison Cook of Reese Marketos.
The Securities and Exchange Commission (SEC) awarded more than $17 million to a whistleblower represented by Whistleblower Law Collaborative’s Suzanne Durrell and Bob Thomas. The whistleblower client submitted a tip under the SEC Whistleblower Program. The tip and subsequent information and assistance led to monetary sanctions in an SEC enforcement action and a related action. The SEC awarded our client 30% of the monetary sanctions collected, the highest percentage award allowed under the SEC Whistleblower Program.
[This] award underscores the SEC’s commitment to rewarding meritorious whistleblowers who provide valuable information and exemplary cooperation that advance the agency’s enforcement efforts,
–Creola Kelly, Chief of the SEC’s Office of the Whistleblower in announcing the award.
Attorneys Bob Thomas and Suzanne Durrell emphasized: “We and our client are very gratified that the SEC recognized and rewarded the extraordinary contributions of our client. We applaud the SEC for its impressive skill and dedication in prosecuting this matter, and for its highly successful track record in working with whistleblowers and their attorneys.”
The SEC program operates somewhat differently than the False Claims Act and other qui tam statutes. SEC provides a very helpful graphic on the process: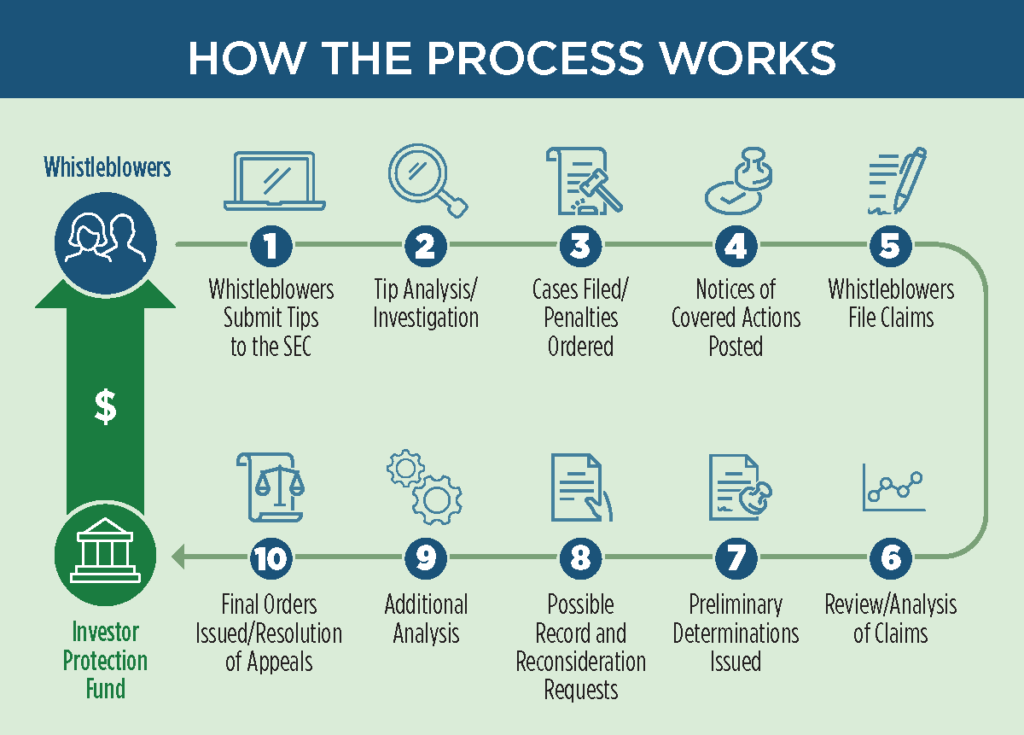
In general, a whistleblower files a “Tip, Complaint, or Referral” form (TCR) with the SEC office of the whistleblower. The SEC then investigates and at its choice may pursue claims based on the tip. SEC periodically posts “notices of covered action.” These notices detail any results potentially subject to whistleblower rewards. Then, whistleblowers must file to claim their share of the recovery. Notably, the program does not give the whistleblower the right to pursue their own claims if the SEC does not.
The SEC may award between 10-30% of the monetary recoveries to an eligible whistleblower. It uses several factors in deciding how much to award. Here, the SEC noted that the highest possible award was appropriate because:
The SEC Whistleblower Program has been very successful. Since the program began, enforcement matters brought using information from meritorious whistleblowers have resulted in orders for nearly $5 billion in total monetary sanctions. This money is returned to the Investor Protection Fund for the benefit of taxpayers, defrauded investors, and others harmed by marketplace misconduct.
Since 2012, the SEC has awarded approximately $1.3 billion to over 275 individual whistleblowers. Importantly, the SEC provides all awards from the Investor Protection Fund. As a result, no money is taken or withheld from harmed investors to pay whistleblower awards.
Whistleblowers may be eligible for an award when they voluntarily provide the SEC with original, timely, and credible information that leads to a successful enforcement action. Whistleblower awards can range from 10 percent to 30 percent of the money collected when the monetary sanctions exceed $1 million.
Further, as in this matter, the SEC protects the confidentiality of whistleblowers and does not disclose any information that could reveal a whistleblower’s identity.
The Whistleblower Law Collaborative has secured awards for clients in several SEC whistleblower cases. It also represents whistleblowers in ongoing SEC investigations.
Whistleblower Law Collaborative LLC, based in Boston, devotes its practice entirely to representing clients nationwide in bringing actions under the federal and state whistleblower laws and programs, False Claims Acts and other whistleblower programs. We have extensive experience representing whistleblowers in False Claims Act and SEC matters.
If you are considering submitting a tip, complaint, or referral to the SEC or are aware of other types of fraud, contact us for a free, confidential consultation.
In July 2022, the United States, Massachusetts, and Connecticut settled a False Claims Act case that our client brought against BioReference Health, LLC. BioReference is one of the largest clinical laboratories in the United States. The case alleged that BioReference paid kickbacks to physicians to induce referrals for its laboratory tests.
In announcing the settlement, United States Attorney Rachael S. Rollins said:
Medical decisions by doctors should be based on what is best for each patient, not a doctor’s personal financial interest. When companies violate the federal health care laws that are meant to protect patients, health care costs for hard working people increase. We will continue to find fraud and use the False Claims Act to make companies that break the law pay back the taxpayers they defrauded as well as pay a financial price for their misconduct.
Under the terms of the False Claims Act settlement, the defendants will pay $10 million, plus interest. BioReference also has entered into a Corporate Integrity Agreement with the HHS Office of Inspector General (HHS-OIG).
The government alleged that BioReference induced physician practices to send their laboratory business to BioReference by paying them above-market rent. The payments were for BioReference to lease space for its Patient Service Centers (“PSCs”). PSCs are locations where BioReference collects patients’ blood samples for testing. These excessive lease payments violated the Physician Self-Referral Law (commonly known as the Stark Law) and the Anti-Kickback Statute (“AKS”). As the United States Attorney’s Office explained in its press release, “Both the Stark Law and the Anti-Kickback Statute are intended to ensure that physicians’ medical judgments are not compromised by improper financial inducements.” Because of these improper payments, BioReference submitted or caused the submission of false claims for payment to federal healthcare programs.
Defendants admitted that, for several years, BioReference made lease payments to physicians and physician practices that exceeded fair market value. Defendants further admitted that, in deciding whether to open, maintain, or close PSCs, BioReference analyzed referrals from nearby healthcare providers. Significantly, BioReference considered referrals from many of the physician-lessors who received excessive rent payments. Finally, defendants conducted internal audits between 2017 and 2019 that identified excessive lease payments to some physician-lessors. However, they failed to report or return any overpayments to government health care programs.
Our client provided government prosecutors with information about the alleged fraud. In 2019, we filed a qui tam complaint under federal and state False Claims Acts. Under the False Claims Act, a private citizen (known as a “relator”) who suspects or knows of fraud against the government can act as a whistleblower and file a sealed complaint on behalf of the government. For successful cases, the government pays a share – between 15% and 30% – to the relator. In this case, our client will receive 17 percent of the recovery.
In DOJ’s press release, the Federal Bureau of Investigation thanked our client, stating:
Laboratories that scheme to enrich their businesses through health care fraud—such as by paying kickbacks—drive up health care costs for everyone. This settlement shows how seriously the FBI takes its responsibility to weed them out, and we’d also like to thank the whistleblower in this case for helping us ensure these entities are held accountable.
-Joseph R. Bonavolonta, Special Agent in Charge of the FBI Boston Division
As this case illustrates, whistleblowers are a critical part of fraud enforcement. Last year, according to DOJ, qui tam cases resulted in over $1.6 billion in False Claims Act recoveries. DOJ noted in announcing this settlement that the False Claims Act is one of government’s most powerful tools to combat healthcare fraud.
The Whistleblower Law Collaborative LLC, based in Boston, devotes its practice entirely to representing clients nationwide in bringing actions under the federal and state False Claims Acts and other whistleblower programs. Among the firm’s many successes is a $234 million settlement earlier this year with Mallinckrodt under federal and state False Claims Acts for Medicaid rebate fraud.
This settlement among the United States, the States, and pharmaceutical companies Mallinckrodt plc and its subsidiary Mallinckrodt ARD LLC resolved allegations that Mallinckrodt violated the federal and state False Claims Acts by knowingly underpaying Medicaid rebates for its high-priced drug Acthar. The total settlement amount is $233,707,865 (plus interest). The United States will receive $123,642,146, and States will receive $110,065,718.
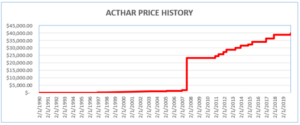
Mallinckrodt knowingly misreported Acthar’s base Average Manufacturer Price (“base AMP”) from January 2013 through June 2020. By doing so, it reduced the rebates it paid to the Medicaid Drug Rebate Program (MDRP) by approximately $650 million. Mallinckrodt had increased Acthar’s price from approximately $50 per vial in 2001 to almost $40,000 per vial.
Where a drug’s price is increased above the rate of inflation, manufacturers must pay an additional rebate. To avoid meeting its increased rebate obligations, Mallinckrodt began reporting Acthar’s base AMP as if it had been approved in 2010 (after the enormous price increases). Acthar, however, had been approved in 1952.
Our client, James Landolt, served as Mallinckrodt’s Director of Internal Controls, Gross to Net Accounting and Government Reporting from November 2015 until July 2017. In that position, he learned that Mallinckrodt had been misreporting the base AMP for Acthar and had underpaid the MDRP by hundreds of millions of dollars.
Mr. Landolt resigned from Mallinckrodt in 2017 and filed a qui tam action in 2018 alleging that Mallinckrodt’s knowing failure to pay correct rebates for Acthar violated federal and state False Claims Acts. In March 2020, the United States intervened in his lawsuit. In June 2020, twenty-eight states, the District of Columbia, and Puerto Rico also intervened.
While the False Claims Act was still under seal, Mallinckrodt sued the Center for Medicare and Medicaid Services (CMS) in federal court in the District of Columbia. Mallinckrodt sought a ruling that it was correctly reporting Acthar’s base AMP and did not have to comply with instructions from CMS to correct its reporting and pay what it owed. In March 2020, the District Court rejected Mallinckrodt’s argument. Two months later, it rejected Mallinckrodt’s motion for reconsideration and for a preliminary injunction.
In October 2020, Mallinckrodt filed for bankruptcy, which stayed the pending False Claims Act case. On March 2, 2022, the bankruptcy court confirmed Mallinckrodt’s plan of reorganization, which included this $234 million settlement.
As part of the settlement, Mallinckrodt entered into a five-year Corporate Integrity Agreement with HHS-OIG that requires, among other things, an independent review organization to annually review multiple aspects of the company’s practices relating to the Medicaid Drug Rebate Program. Mallinckrodt began reporting the correct base AMP for Acthar in June 2020 after losing its case in United States Court in D.C.
Mr. Landolt will receive a 20% share of amounts paid under the federal and state False Claims Acts. Whistleblowers like Mr. Landolt are critical in the fight against fraud. Under the False Claims Act, a private citizen-relator who suspects or knows of fraud against the government can file a sealed complaint on behalf of the government. In successful cases, the relator is entitled to a share of the government’s recovery.